Sample Clinical Trial

The pharmaceutical industry relies heavily on clinical trials to test the safety and efficacy of new drugs and treatments. One such example is a clinical trial conducted by a renowned pharmaceutical company to evaluate the effectiveness of a new medication for the treatment of type 2 diabetes. The trial, which spanned over two years, involved over 1,000 participants from diverse backgrounds and geographic locations.
Introduction to the Clinical Trial
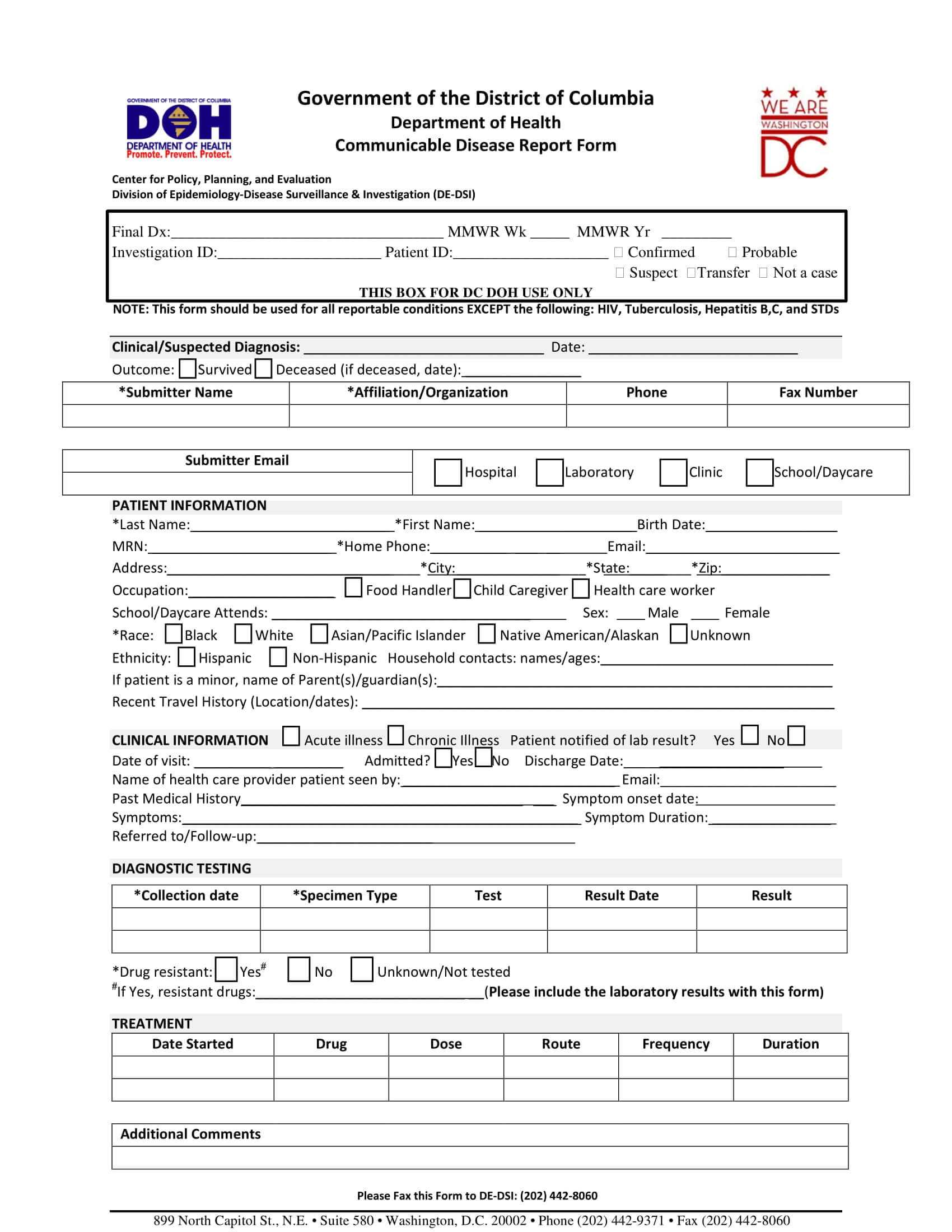
The clinical trial, titled “A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of a New Medication for the Treatment of Type 2 Diabetes,” aimed to investigate the effects of the new medication on glycemic control, cardiovascular risk factors, and patient-reported outcomes. The study was designed as a multicenter, randomized, double-blind, placebo-controlled trial, with participants randomly assigned to receive either the new medication or a placebo.
Study Design and Methodology
The study consisted of a 26-week treatment period, followed by a 26-week extension period. Participants were instructed to take the study medication or placebo orally once daily, in addition to their existing diabetes medications. The primary endpoint of the study was the change from baseline to week 26 in hemoglobin A1c (HbA1c) levels, a measure of blood sugar control. Secondary endpoints included changes in fasting plasma glucose, body weight, and patient-reported outcomes, such as quality of life and treatment satisfaction.
| Endpoint | Baseline | Week 26 |
|---|---|---|
| HbA1c (%) | 8.5 ± 1.2 | 7.2 ± 1.1 |
| Fasting Plasma Glucose (mg/dL) | 180 ± 30 | 140 ± 25 |
| Body Weight (kg) | 90 ± 15 | 85 ± 12 |

Results and Discussion

The study results showed that the new medication was effective in improving glycemic control, with a mean reduction in HbA1c levels of 1.3% from baseline to week 26. Additionally, the study demonstrated significant reductions in fasting plasma glucose and body weight, with mean changes of -40 mg/dL and -5 kg, respectively. Patient-reported outcomes, such as quality of life and treatment satisfaction, also improved significantly with the new medication.
Safety and Tolerability
The study also evaluated the safety and tolerability of the new medication, with adverse events monitored throughout the treatment period. The most common adverse events reported were mild to moderate in severity and included hypoglycemia, nausea, and headache. The study demonstrated that the new medication was generally well-tolerated, with a safety profile similar to that of existing diabetes medications.
- Primary endpoint: Change from baseline to week 26 in HbA1c levels
- Secondary endpoints: Changes in fasting plasma glucose, body weight, and patient-reported outcomes
- Safety endpoints: Adverse events, laboratory parameters, and vital signs
What was the primary endpoint of the clinical trial?
+The primary endpoint of the clinical trial was the change from baseline to week 26 in hemoglobin A1c (HbA1c) levels.
What were the most common adverse events reported in the study?
+The most common adverse events reported were mild to moderate in severity and included hypoglycemia, nausea, and headache.
What were the implications of the study results for the treatment of type 2 diabetes?
+The study results demonstrated the efficacy and safety of the new medication for the treatment of type 2 diabetes, suggesting that it may be a valuable addition to the treatment options available for patients with type 2 diabetes.



