Tryptophan Hydrogen Bonding Explained
Tryptophan, an essential amino acid, plays a crucial role in various biological processes, including protein structure and function. One of the key aspects of tryptophan's behavior is its ability to form hydrogen bonds, which are essential for stabilizing protein structures and facilitating molecular interactions. In this article, we will delve into the world of tryptophan hydrogen bonding, exploring its principles, mechanisms, and significance in biological systems.
Introduction to Tryptophan and Hydrogen Bonding

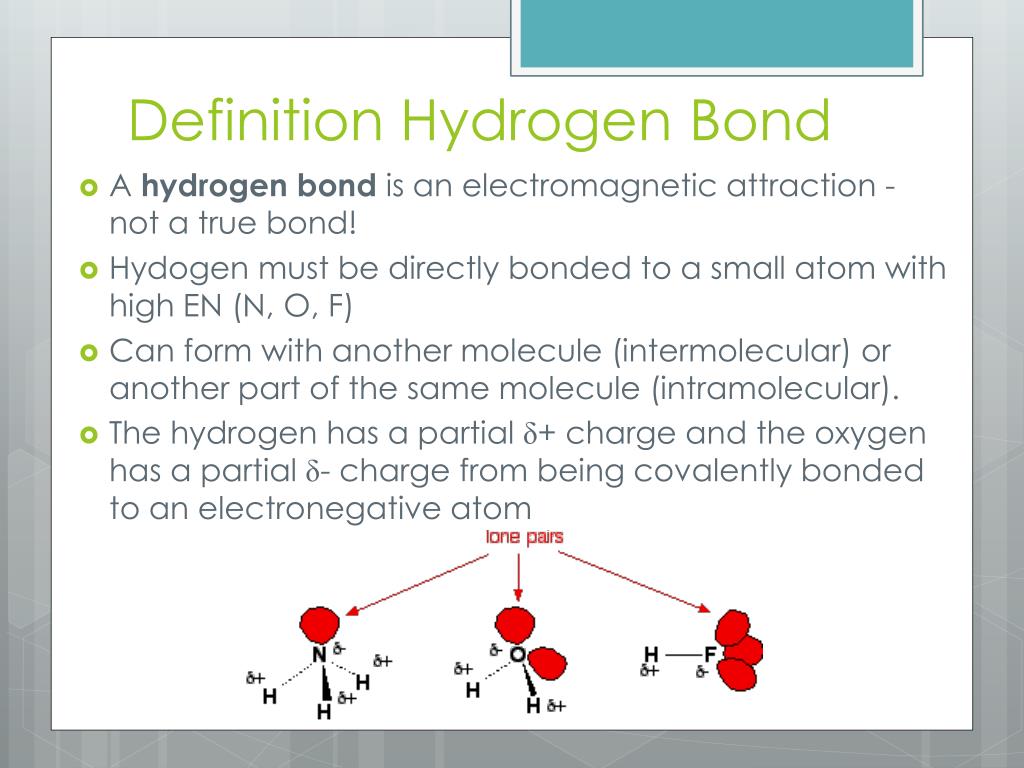
Tryptophan, with its distinctive indole side chain, is the largest and most hydrophobic of the 20 standard amino acids. Its unique structure, featuring a planar, aromatic ring system, enables it to engage in various non-covalent interactions, including hydrogen bonding. Hydrogen bonds are relatively weak electrostatic attractions between atoms, typically involving hydrogen atoms bonded to highly electronegative atoms such as oxygen, nitrogen, or fluorine. These interactions are vital for maintaining protein stability, facilitating molecular recognition, and modulating biochemical reactions.
Principles of Hydrogen Bonding in Tryptophan
The tryptophan side chain, comprising an indole ring and a methylene group, offers multiple sites for hydrogen bonding. The indole nitrogen (N1) and the carbonyl oxygen of the peptide backbone can act as hydrogen bond acceptors, while the indole C-H groups and the amino group of the peptide backbone can serve as hydrogen bond donors. The planarity of the indole ring also enables tryptophan to participate in π-π stacking interactions, which can complement or compete with hydrogen bonding.
| Type of Hydrogen Bond | Donor | Acceptor |
|---|---|---|
| Conventional hydrogen bond | N-H or O-H | O, N, or F |
| Weak hydrogen bond | C-H | O, N, or F |
| π-π stacking interaction | Aromatic ring | Aromatic ring |

Mechanisms of Tryptophan Hydrogen Bonding

The mechanisms underlying tryptophan hydrogen bonding are complex and multifaceted. In proteins, tryptophan residues often participate in hydrogen bonding networks that involve other amino acids, water molecules, or ligands. These networks can stabilize protein structures, modulate protein function, and influence protein-ligand interactions. The planarity of the indole ring also allows tryptophan to engage in π-π stacking interactions, which can complement or compete with hydrogen bonding.
Role of Tryptophan Hydrogen Bonding in Biological Systems
Tryptophan hydrogen bonding plays a crucial role in various biological processes, including protein folding, enzyme catalysis, and protein-ligand recognition. In protein folding, tryptophan residues can participate in hydrogen bonding networks that stabilize the protein structure. In enzyme catalysis, tryptophan residues can engage in hydrogen bonding interactions with substrates or cofactors, facilitating chemical reactions. In protein-ligand recognition, tryptophan residues can participate in hydrogen bonding interactions with ligands, modulating binding affinity and specificity.
- Protein folding: Tryptophan hydrogen bonding stabilizes protein structure
- Enzyme catalysis: Tryptophan hydrogen bonding facilitates chemical reactions
- Protein-ligand recognition: Tryptophan hydrogen bonding modulates binding affinity and specificity
Future Implications of Tryptophan Hydrogen Bonding Research
Research on tryptophan hydrogen bonding has significant implications for our understanding of protein structure and function, as well as the development of novel therapeutic strategies and biomimetic materials. By elucidating the principles and mechanisms of tryptophan hydrogen bonding, scientists can design more effective inhibitors of enzyme activity, develop more potent and selective ligands for protein targets, and create novel biomimetic materials that mimic the properties of biological systems.
What is the significance of tryptophan hydrogen bonding in protein structure and function?
+Tryptophan hydrogen bonding plays a crucial role in stabilizing protein structures, facilitating molecular interactions, and modulating biochemical reactions. Its ability to form multiple types of hydrogen bonds and engage in π-π stacking interactions makes it a versatile amino acid in protein structure and function.
How does tryptophan hydrogen bonding contribute to enzyme catalysis?
+Tryptophan hydrogen bonding can facilitate enzyme catalysis by engaging in hydrogen bonding interactions with substrates or cofactors, positioning them for optimal chemical reaction. The planarity of the indole ring also allows tryptophan to engage in π-π stacking interactions, which can complement or compete with hydrogen bonding.
What are the potential applications of tryptophan hydrogen bonding research?
+Research on tryptophan hydrogen bonding has significant implications for the development of novel therapeutic strategies and biomimetic materials. By elucidating the principles and mechanisms of tryptophan hydrogen bonding, scientists can design more effective inhibitors of enzyme activity, develop more potent and selective ligands for protein targets, and create novel biomimetic materials that mimic the properties of biological systems.

